
 Address of Medical Laboratory:
Address of Medical Laboratory: Customer Service Email:
Customer Service Email: Service Hotline:
Service Hotline: TEL:
TEL: Website:
Website:
YuceNeo provides customers with a wide selection of services, including biomarker research for clinical trials of neoantigens, high-quality neoantigen screening and validation, in vivo and in vitro immunogenicity validation, peptide synthesis and preparation, and dynamic monitoring of the efficacy of clinical trials.
Neoantigen Prediction and Validation
▪ YuceNeo has its own negative and positive neoantigen databases that are independently developed and experimentally validated;
▪ Based on the "Ladder for Cancer Moonshot", the earliest real-world tumor immunotherapy database in China, a shared neoantigen database has been established;
▪ Its independently developed TruNeo™ intelligent machine learning algorithm incorporates multiple factors in the biological pathways of neoantigen presentation and recognition, making the test of neoantigens more accurate;
▪ The evaluation data of the Tumor Neoantigen Selection Alliance (TESLA) showed that TruNeo™ has a sensitivity of 87.5% for neoantigen prediction, ranking first among all teams participating in the review, and the validation rate of the top 10 neoantigens reaches 40%, ranking among the top 4 participating teams
Validation of the immunogenicity of neoantigen peptides
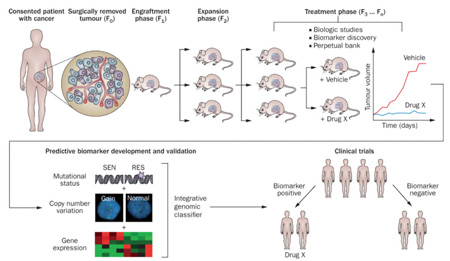
The specimens used for transplantation of PDTX (Patient-Derived Tumour Xenografts) in humanized mice are directly derived from human tumor tissues and have not been cultured in vitro, stably retaining the genetic, histological, and phenotypic characteristics of the tumor, i.e., tumor heterogeneity. Therefore, PDTX can be used to screen the sensitivity or resistance of immune drugs, and the test results have good clinical predictability.
In addition, because PDTX better retains the tumor mesenchymal and stem cell components during the transplantation process, the microenvironment for tumor growth is closer to the actual situation. PDTX can also provide a large number of specimens for the preservation and passage of tumor samples, which can be used before clinical treatment for the efficacy validation of the immunogenicity of cells or vaccine preparations. It can also be used to determine the appropriate sample size and efficacy evaluation for small preclinical trials.
Nat Rev Clin Oncol.2012;9(6):338–350. Published 2012 Apr17. doi:10.1038/nrclinonc.2012.61
The figure shows the operation procedure of PDTX. Obtain fresh surgical tissues, cut it into small pieces, and then implant the tissues into the subcutaneous region or ventral side of immunodeficient mice. Mice containing patient-derived materials are called generation F0, and mice of subsequent generations are numbered consecutively (F1, F2, F3, etc.). Generally speaking, the third generation can be used for drug therapy, but it is mainly determined by the degree of difference between PDTX and the patient's tumor in terms of genetics and histology.
The PDTX test can be used to predict and validate the efficacy of neoantigen drugs in advance, improving the effectiveness rate of personalized tumor neoantigen therapy.
In vitro validation of immunogenicity by EliSpot test
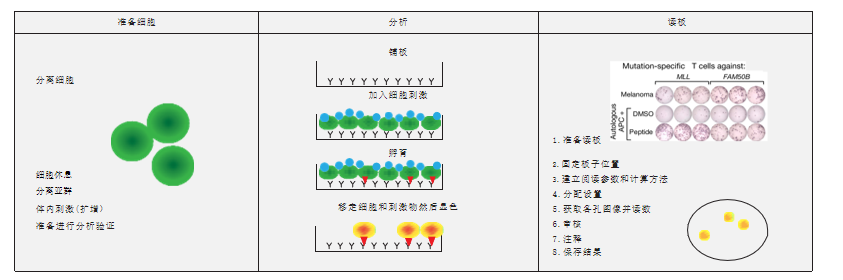
In vitro immunogenicity validation technology—EliSpot
In organisms, cells will produce cytokines (such as IFN-γ) after stimulation. These cytokines will be captured by specific antibodies and then develop colors to form spots. The number of spots represents the level of immunogenicity of the corresponding neoantigen. This platform will further enhance the accuracy of neoantigen screening at YuceNeo. EliSpot has little effect on the biological process of cells, and higher sensitivity in cell detection. Based on test on the single-cell level, one IFN-γ-secreting cell can be detected from 200,000 to 300,000 cells through the EliSpot test. EliSpot is more sensitive than Elisa and the limiting dilution method.
In vitro validation of immunogenicity by NESt
EliSpot can be used in vitro to analyze which neoantigens can stimulate T cells to secrete cytokines, but it cannot detect the TCR of tumor-specific T cells. YuceNeo's unique NESt (Neoantigen Expansion of Specific T Cells) validation technology integrates TCR sequencing of peptide-stimulated T cell culture with a biological information platform to identify antigen-specific clonal amplification.
The NESt platform of YuceNeo compares the TCR databases with and without stimulation by neoantigen peptides. If a TCR with an increasing frequency can be found, it can indicate that the corresponding neoantigen is immunogenic.
The NESt technology platform not only can validate the immunogenicity of neoantigens screened by TruNeo™ in a high-throughput manner, but also can be used to dynamically monitor the effect of tumor immunotherapy*.
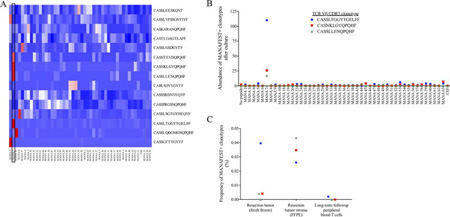
Figure: The NESt analysis can identify multiple recognized neoantigens and provide TCR sequences to track anti-tumor immune responses in tissues and peripheral blood.
Immune Repertoire (IR) refers to the sum of all functional B cells and T cells in the circulatory system of an individual at any given time. The receptors used to recognize antigens are B cell receptor (BCR) and T cell receptor (TCR). There is a region in BCR/TCR called Complementary Determining Region (CDR), including CDR1, CDR2, and CDR3. Among them, CDR3 is the most variable and plays a key role in antigen recognition. Therefore, CDR3 gene sequence can be used as a unique molecular identifier for the diversity of the immune repertoire.
Different clones of B/T lymphocytes have CDR3 genes with different sequences or lengths, and can be translated into different CDR3 polypeptide sequences, reflecting the restoration and reconstruction of the diversity of BCR/TCR immune repertoire of B/T cells. The assessment and measurement of the diversity of the immune system provide a quantitative indicator of health status. In the immune repertoire research, the genes or RNA molecular sequences of BCR and TCR in the immune system are determined, and the codes are analyzed by using high-throughput sequencing technology. CDR3 is found by using the position information of sequence alignment and the pattern recognition method, and its clonality and polymorphism are calculated to finally output the results in tabular and graphical formats.
In addition, relying on the NESt assay platform of YuceNeo, the comparison of TCR data obtained with and without neoantigen peptide stimulation and the changes in the expression of TCR sequences in different treatment stages can track and evaluate the efficacy of neoantigen-related immunotherapy. TCR-seq can be used not only to detect the antigen-specific response after 10 days of peptide stimulation, but also to quantitatively analyze the polyclonal nature of the response.
YuceNeo's dynamic monitoring platform of personalized neoantigen therapy combines the immune repertoire and the NESt technology to achieve the full dynamic monitoring and management of the personalized clinical trial of tumor neoantigens, and help to improve the post-cure performance of clinical trials of tumor immunotherapy.
Cancer Immunol Res 2018; 6:888-899. Published Online First June 12, 2018.
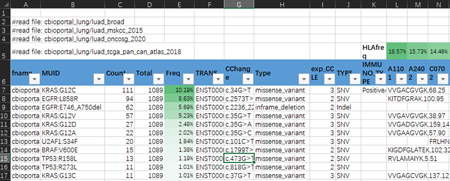
Data sources for shared neoantigen databases:
Data collection: Four open research cohorts of lung adenocarcinoma (LUAD), including TCGA, etc.
Format explanation:
Filtering: Mutation frequency (N ≥ 2) && HLA affinity (< 500 nm) && HLA population frequency (> 2%) && exp_CCLE > 1
Sorting: Mutations and HLA are sorted in the descending order by population frequency, respectively
Recording: Short peptide sequence with the highest affinity for MHC-I
Pending validation: Immunogenicity (IMMUNO_TYPE)
Other research cohorts:
LUSC (lung squamous cell carcinoma), STAD (gastric cancer), COAD (colorectal cancer), and Esophage
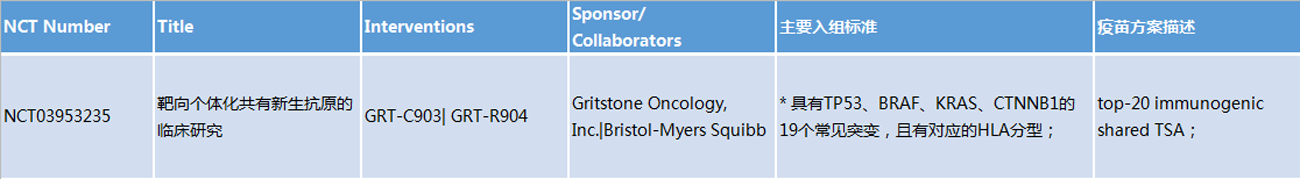
2) Combination protocol of shared neoantigens
a. Several clinical trials have mentioned methods to find shared neoantigens from the research cohorts:
Early research of Nous-209 found a large number of recurring frameshift mutations in the MSI-H cohort.
The patent obtained from the clinical trial of Gritstone based on 19 common mutations also mentions that the antigen combination protocol reduces autoimmunity.
b. The characteristics of protocols of shared neoantigens in clinical trials:
Number of neoantigens: A single gene (such as BRAF V600E), or a combination of 20 antigens, or a combination of more than 200 frameshift mutations;
HLA typing: HLA typing corresponding to the mutation, or a small number of high-frequency HLA typing, or neoantigen targeting MHC-II typing.
(Please contact customer service staff for more data)
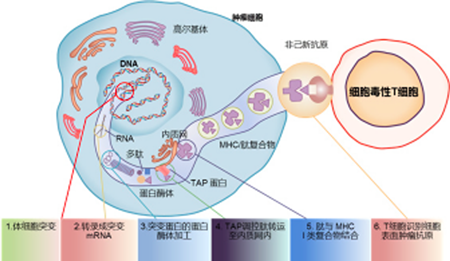
Neoantigens are tumor-specific antigens produced by mutated genes in tumor cells that are not expressed by normal cells and can cause T cell activation. In cells, neoantigens can be degraded into short peptides (antigenic epitopes), which bind to MHC-I or MHC-II molecules with high affinity to form a complex, and are presented to the cell surface by MHC molecules in the form of the complex. After treating the complex as a "non-self" substance, the T cells are activated to become effector T cells, which then begin to attack and eliminate tumor cells. Tumor antigens are classified into tumor-associated antigens (TAA) and tumor specific antigens (TSA). TAAs are expressed not only in tumors, but also in normal tissues. Due to central tolerance, their antigen recognition affinity with T cells is generally low. In traditional adoptive cell therapy and tumor vaccines, TAAs are mainly used for the experimental design, which often faces problems such as poor efficacy and strong side effects. Neoantigens are TSAs with strong immunogenicity and are considered to be an ideal choice for immunotherapy in the industry.
Chabanon RM,etal.Clinical Cancer Research,2016.
The Tumor Neoantigen Selection Alliance (TESLA) is composed of more than 30 neoantigen research institutions, including the famous Cancer Research Institute (CRI), the Parker Institute of Cancer Immunotherapy (PICI), the Memorial Sloan Kettering Cancer Center (MSKCC), and MD Anderson Cancer Center in the US. It aims to predict neoantigens through artificial intelligence algorithms, and ultimately produce more effective and targeted cancer vaccines tailored for each patient.
The scientists of Tumor Neoantigen Selection Alliance (TESLA), initiated by the Parker Institute of Cancer Immunotherapy (PICI) and Cancer Research Institute (CRI), have discovered the algorithm model and core parameters that can better predict which neoantigens can activate the function of cancer treatment. TESLA brings together 36 top biotechnology, pharmaceutical, academic, and non-profit scientific research teams including YuceBio, and the research results were finally published in the Cell magazine, which will undoubtedly give birth to a new generation of more effective and more personalized cancer immunotherapy. It also represents that a new era of personalized immunotherapy based on neoantigens has arrived.
When the scientists tested another set of the data model from cancer samples emphasizing these five characteristics, it could accurately predict 75% of effective neoantigen targets and filter out 98% of invalid new targets.
Through computational analysis, TESLA found five characteristics that strongly indicate which cancer markers are most likely to produce an immune response. These characteristics can be divided into two categories: the presentation mode of neoantigens on tumor cells and how the immune system recognizes neoantigens.
Cooperative clients include the DC vaccine project of Academician Wei Yuquan of West China Hospital involving more than 500 cases of non-small cell lung cancer, the TILs treatment for cervical cancer by Academician Ma Ding of Wuhan Tongji Hospital, bile duct cancer project of Shanghai Cell Group, and gastrointestinal tumor project of Shanghai Changhai Hospital, etc. Among them, 5 projects have entered the clinical stage, making the Company the earliest and leading service provider in the field of neoantigens in China.
Take the area of lung cancer as an example:
1. Adjuvant cancer treatment with neoantigen-based DC vaccines (West China Hospital)

Case 1:
A 75-year-old male with brain metastatic small cell lung cancer experienced resistance to multiple chemotherapeutics and was subsequently treated with anti-PD-1 therapy, but the disease recurred.
WES and RNA sequencing was used to assess tumor mutations of the patient and predict potential neoantigens. 12 neoantigen peptides were selected to synthesize the autologous DC neoantigen vaccine. 1.5 × 10^8 units of DC vaccine were subcutaneously infused into the patient to evaluate the efficacy. Ten months after the vaccine treatment, objective response to brain metastases was observed. The IFN-γ ELISpot was used to analyze the response of CD4+ and CD8+ T cells in the blood to 12 neoantigen epitopes before and after treatment, so as to evaluate the immunogenicity of each epitope. Among the predicted neoantigen epitopes, 75% of the new epitopes caused an immune response of CD4+/CD8+ T cells, indicating that in this patient, T cells responded to multiple predicted new epitopes.
Case 2:
Research objective: To evaluate the efficacy of neoantigen vaccine combined with ICI in a patient with advanced squamous cell lung carcinoma
Case: A 68-year male patient with advanced squamous cell lung carcinoma who has received chemotherapy, radiotherapy, targeted therapy, and anti-angiogenic therapy
Method: Whole exome sequencing, mutation test, prediction of neoantigens; selection of 17 neoantigen peptides for the synthesis of the autologous DC vaccine. 1.38 × 108 units of DC vaccine were injected into the patient (days 1, 7, 15, 22, and 30) in combination with anti-PD-1 antibody (4 mg/kg, followed by 2 mg/kg biweekly)
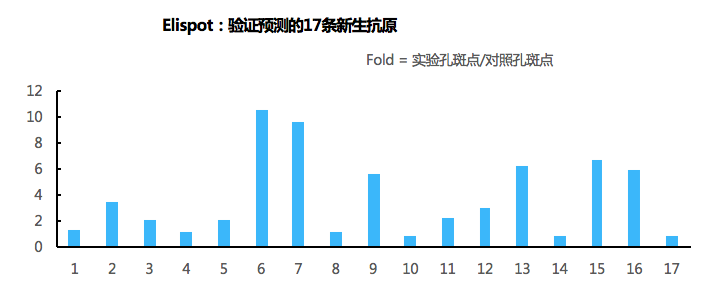
The immunogenicity of each of the 17 neo-epitopes was analyzed by IFN-γ ELISpot in CD4+ and CD8+ T cells in blood samples.
Results:
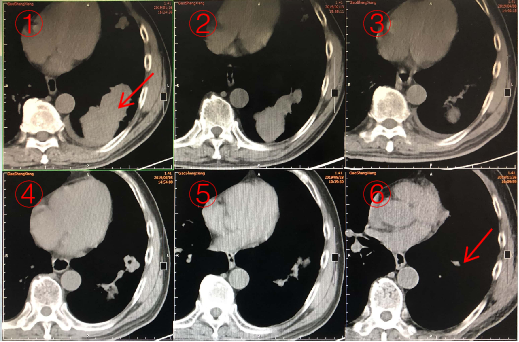
The patients had a partial response after treated with vaccine for 10 months.
64.71% (11/17) of the predicted neo-epitopes caused an immune response in CD4+/CD8+ T cells.
Conclusion:
The DC-based neoantigen vaccine combined with PD-1 inhibitor is safe and well tolerated in lung cancer patients who failed with multi-line treatments.
The neoantigen vaccine can cause T cell immune response in vitro.
The bioinformatic prediction algorithm helps select high-quality neoantigens.
This study introduces a framework for testing combination therapy of the neoantigen vaccine in patients with advanced lung cancer.
Case 3:
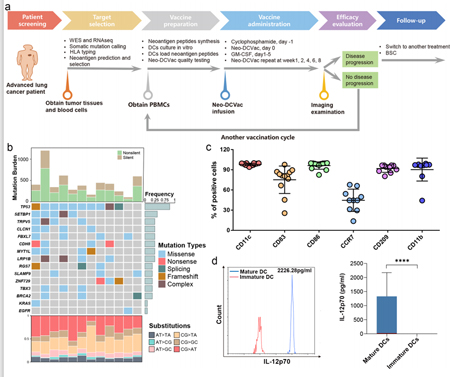
A total of 21 patients with advanced lung cancer who failed with multi-line treatments were enrolled, 12 of which were eligible for vaccine treatment:
Neoantigens were predicted and selected via WES + RNA sequencing;
Autologous PBMCs were cultured in vitro and induced to differentiate into DCs, and the synthetic neoantigen peptides were loaded in DCs to prepare the DC-based neoantigen vaccine;
The vaccine was given subcutaneously and efficacy was evaluated, and vaccine treatment was repeated after each cycle (including 5 doses) in case of no disease progression, while other treatments were applied in case of disease progression.
Conclusion:
T53 mutation occurs at the highest rate in patients and only one patient carries EGFR mutation.
The average proportion of DC cells expressing mature DC phenotypic markers and antigen presentation markers is high in the manufactured DC-based vaccines.
Copyright©2021
Shenzhen yucebio Technology Co., Ltd. all rights reserved
Yueicp no.16128839

