
 Address of Medical Laboratory:
Address of Medical Laboratory: Customer Service Email:
Customer Service Email: Service Hotline:
Service Hotline: TEL:
TEL: Website:
Website:
YuceNeo is honored to work with our partners in pharmaceutical, biotechnology and in vitro diagnostic sectors on companion diagnostic (CDx) projects.
YuceNeo focuses on the research and development of biomarkers associated with tumor immunotherapy. The biomarkers are used to identify target patients who are more likely to benefit from a specific tumor immunotherapy, which considerably accelerates the development and approval of drugs, as well as facilitates the co-development of CDx products to accurately and reproducibly select patients who will benefit from a specific tumor immunotherapy. YuceNeo has gained experience in over 50 clinical projects and has collaborated with pharmaceutical, biotechnology and IVD companies on more than 20 projects. It plays an important role in pioneering new ways of developing anti-cancer drugs.
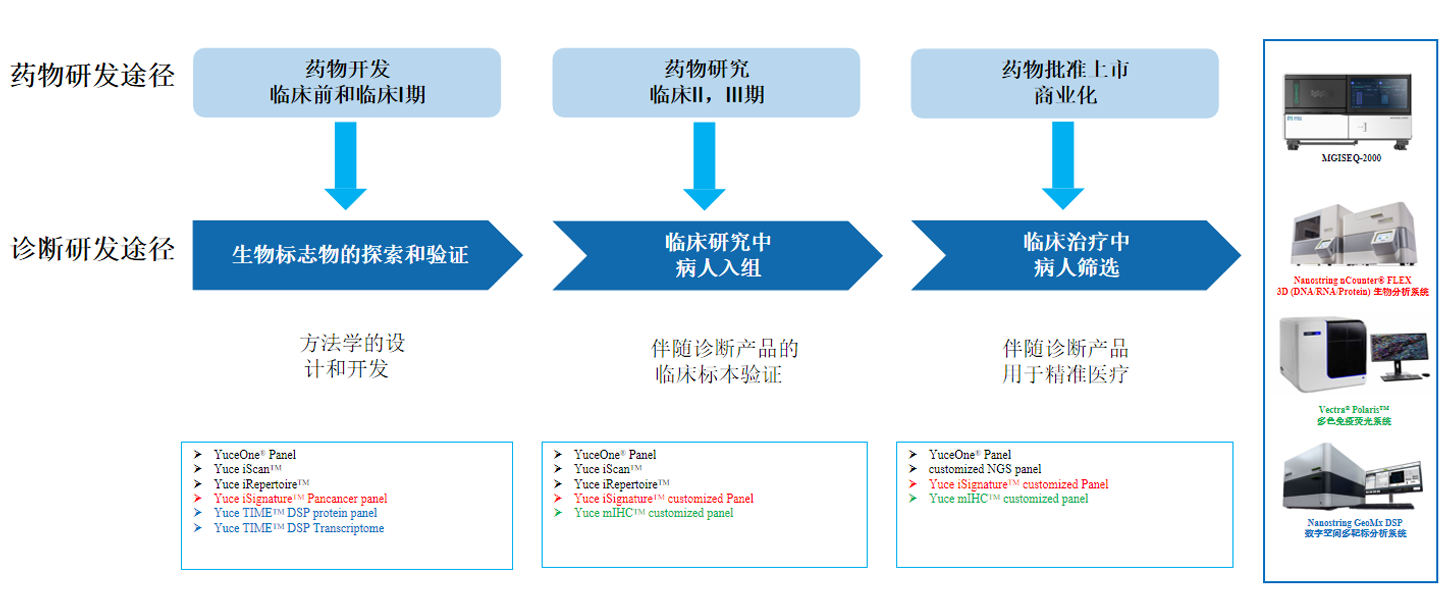
The development of CDx products is complicate and time-consuming. YuceNeo offers one-stop services for the development of CDx products, including consulting, customization, commercialization, and integration. Our experts in experimental research, bioinformatics, pathology, and business development will support in the development of CDx products.




Whole exome sequencing (WES) and RNA-seq evaluate tumor tissues based on DNA and RNA respectively. WES + RNA-seq can comprehensively predict high-quality neoantigen sequences and accurately classify the population benefiting from the immunotherapy, targeted therapy or combination therapy. The effectiveness, sensitivity, resistance, and drug risk of targeted therapy, chemotherapy, immunotherapy and personalized cell therapy are integrally assessed. Further assessment of immunotherapy efficacy can also be performed in combination with Yuce in-house Database, using Yuce's OTMB, ITH, IO-TNL, and other algorithms.
YuceOne ® Plus covers 757 tumor neoantigen-related genes, 339 microsatellite instability (MSI) sites, 74 targeted detection genes, 31 chemotherapy drug-related genes, predict high quality neoantigen sequence, accurate classification of immunotherapy, targeted therapy or combined treatment beneficiaries, comprehensively evaluate the effectiveness, chemotherapy, immunotherapy and personalized cell therapy of targeted therapy, sensitivity, resistance and drug risk. In addition, the use of OTMB, ITH, IO-TNL can be further evaluated in combination with the Yuze free database and other algorithms to evaluate the efficacy of immunotherapy. The Yuzer biological TMB CDx detection kit enters the registration certification stage and can develop the finest genome-level immune diagnostic biomarker. for the TMB CDx cooperation.
YuceOne ® Plus (NI) accurately divides the groups benefiting from immunotherapy, targeted therapy, or combined therapy, and comprehensively evaluates the efficacy, sensitivity, resistance, and medication risk of targeted therapy, chemotherapy, immunotherapy, and personalized cell therapy. The 1.4M-size Panel and specific machine learning model, designed based on genomic big data at home and abroad, yields a high correlation between bTMB and tTMB, and clinical sample sequencing data shows a correlation of 0.90. MSI state (bMSI) in ctDNA, and b MSI values have 80% sensitivity and 100% specificity in comparison with tissue MSI detection results.
Immune Repertoire is the sum of all functional diversity B and T cells in an individual at any specified time, and is an general and historical record of an individual's immune function. Mainly applied to:
1. leukemia / myeloma / lymphoma MRD test
2. slow-lymphoid leukemia / lymphoma IGHV mutation detection
3. cancer immunotherapy is accompanied with diagnosis
IO 360 panel is a comprehensive panel, containing 770 genes covering 16 key tumor immune signaling pathways and processes, analyzing 34 feature spectra including Tumor inflammation signature (TIS), 14 feature spectra for detecting immune cell groups and 19 innovative feature spectra for detecting important tumors and immune activity. biomarker for development and verification in early clinical research projects.
CAR-T Panel was created in collaboration with eight leading centers in the CAR-T therapy field, designed for the entire CAR-T R & D production process capable of uniform and robust analysis of white blood cells removal, CAR-T cell manufacturing products and after infusion. The customizable 780 gene expression Panel contains content (Phenotype, Cell Types, TCR Diversity, Activation, Metabolism, Persistence, Exhaustion, Toxicity) for measurement of eight basic components of CAR-T cell biology, including T cell activation, metabolism, depletion and TCR receptor diversity, and can choose a customized gene target (up to 30 targets) or a protein target (up to 10 targets).
Development of the Vectra mIHC Panel for immunohistochemical immune microenvironment assessment for specific tumors based on the Polaris ™ platform:
Breast cancer: CD4,CD8,FOXP3,CD68,ER-a/PR,PD-L1/LAG3/TROP-2/TIM-3;
Lung cancer: CD4,CD8,FOXP3,CD68,AHR/Ki67,PD-L1/LAG3/TROP-2/TIM-3;
Colorectal cancer: CD4,CD8,FOXP3,CD68,Calretinin/MUC2,PD-L1/LAG3/TROP-2/TIM-3;
Esophageal cancer: CD4,CD8,FOXP3,CD68,CTSD/CK5/6,,PD-L1/LAG3/TROP-2/TIM-3;
The specific multi-color immunohistochemical Panel can be customized for customer clinical / research projects, and the relevant cost of customization will be agreed separately.
Yuce TIME ™ GeoMx DSP Protein Panel is designed to provide a flexible and comprehensive assessment of the tumor and tumor microenvironment with a modular design, Up to 96 protein targets were analyzed at spatial resolution from a single tissue slice: the immune cell profile core Panel contains 18 protein targets for immune cell typing; 1 customized target Panel(up to 10 customized targets); 7 modular Panel s (IO Drug Target Module, Immune Activation Status Module, Immune Cell Typing Module, Pan-Tum o r M o d u l e, Cell Death Module, PI3K / AKT Signaling Module, MAPK Signaling Module, Select up to 6 modules for detection).
Yuce TIME ™ DSP Transcriptome has two Cancer Transcriptome Atlas (CTA) and Whole Transcriptome Atlas (WTA) products:
CTA is designed for a comprehensive analysis of tumor biology, tumor microenvironment, and immune responses. RNA expression of more than 1800 genes was analyzed at spatial resolution at any area of interest in a single tissue slice.
WTA is designed for a comprehensive analysis of spatial biology. The RNA expression of more than 18,000 human genes was analyzed at a spatial resolution in any area of interest in a single tissue slice.
Based on the combined use of biomarker detection becomes an inevitable necessity based on multi-factor properties of cancer-immune interactions, the cancer-immune panorama ography is a network framework for tumors interacting with the immune system in individuals. The framework collectively contains the following dimension data:
Heterogenicity of 1) tumor is largely determined by the expression of its neoantigen;
2) routine immune state, can be analyzed through lymphocytes and types;
3) immune cell infiltration state;
4) immune checkpoint deficiency, such as PD-L1 expression;
5) inflammation associated factors deficiency 5);
The deficiency of 6) to inhibit tumor metabolism, such as the excessive LDH(lactate dehydrogenase) concentration in serum, is greatly associated with the poor efficacy of CTLA-4 and PD-L1 inhibitors;
Analysis of the sensitivity of 7) tumor cells to immunoeffectors
Yuce is applying for a large panel product covering 811 gene-related regions (2.3 M), and is in the process of registering 15 CDx loci as well as two markers (MSI and TMB). By now, we have basically completed the development of IVD products for these markers. In addition to the declared intention, this panel is capable of detecting HLA-LOH and neoantigens. Our registration team is involved in the development of TMB testing standards. YuceBio has become a collaborative partner of National Institutes for Food and Drug Control to qualify national TMB standards. Yuce registration team consists of professionals dedicated to experimental R&D, bioinformatics R&D, quality, and registration, with core members having over 10 years of experience in these fields. Our experts also excel in capture chip design, NGS experiments, bioinformatics software development, quality system establishment, and registration regulations. We have achieved early product development, raw material screening, process optimization, analytical performance research, and bioinformatics pipeline standardization. In terms of product reliability, Yuce is leading the industry in China.
Capable of registration and certification for CDx products

Copyright©2021
Shenzhen yucebio Technology Co., Ltd. all rights reserved
Yueicp no.16128839

